Invokana Settlement
(Updated Nov. 8, 2018)
Note: We are no longer taking Invokana and Invokamet cases.
In October 2018, Johnson & Johnson and their subsidiary Janssen agreed to settle a large portion of the 1,000 Invokana lawsuits filed against them in a New Jersey multidistrict litigation (MDL). It’s unclear how many lawsuits the confidential settlement fund will cover.
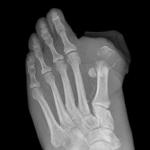
The lawsuits allege that Invokana and Invokamet caused diabetic ketoacidosis, acute kidney injury, and amputation injuries.
The FDA added ketoacidosis warnings to Invokana, Invokamet, Farxiga, and Xigduo XR (all SGLT2 inhibitors) in June 2016—more than three years after Invokana’s March 2013 FDA approval. But though the FDA believed the risk of ketoacidosis to be great enough to warrant a warning, lawsuits ask why didn’t Janssen include the risk on their drug labels from the beginning?
2018—Invokana Confidential Settlement
A motion filed on October 16, 2018 revealed that Johnson & Johnson and its subsidiary Janssen had agreed to establish a confidential settlement fund that would resolve most of the 1,000 lawsuits filed against them in a New Jersey MDL. It's likely the companies will also settle the lawsuits pending in Pennsylvania and California state courts.
$2.4 Billion Settlement for Actos Plaintiffs
Though it’s impossible to say how much relief Invokana plaintiffs might receive from the settlement or in court, it could be a significant amount. For example, in 2015 the Japanese drug company Takeda settled lawsuits over its Type 2 diabetes drug Actos for $2.37 billion. (Actos has been linked to bladder cancer.)
In 2014, a Louisiana jury awarded $6 billion to an Actos user, Terrance Allen, who had developed bladder cancer. (A judge later reduced the award to $38.6 million.)
Injured by a Drug? We Can Help
If you were injured by a pharmaceutical drug, you may be able to file a lawsuit against the manufacturers. Lawsuits can help recover compensation for past and future medical bills, loss of income, pain and suffering, and more.
Our attorneys have taken on the most powerful pharmaceutical giants in the country, including Johnson & Johnson and Bayer, and have the skills and resources to take your case. Contact us today for a free, no-obligation legal review. It never costs a thing unless we win a jury award or settlement for you.